Services
Expert Solutions, Tailored for You! Explore our services designed to drive success.
Need Solutions? We’re Here to Help!
With decades of experience in formulation development, regulatory compliance, process improvement, and facility design, we offer tailored solutions to meet global industry standards.
Turn-Key Projects from Feasibility to Completion.
We have extensive experience in setting up and expanding pharmaceutical, nutraceutical, and cosmetic units.
- Feasibility & Assessment
- Cost estimation
- Product selection
- DRAP submission layouts with zone classification
- Phase-wise expenses
- Utility points details
- Optimal process flow designing
- Assistance in purchasing machinery, materials, and lab equipment
- Machinery and equipment placement and installation
- Recruitment for all critical departments from management to supervision
- Procurement of materials

Mahinery & Equipment Selection

Layout Design

Turn Key Project

Staff Hiring
For Existing Units, we provide:
- Extension & Alteration Plans
- Process automation for enhanced efficiency
- Process Optimization
- Output Enhancement
- Product costing accurate and expert-driven
- Machine need analysis for identifying cost-effective and efficient machinery solutions

Automation

Machine Need Analysis

Process Optimization

Automation
We have led the development of numerous new formulations, including:
- Gel matrix and wax matrix sustained-release tablets for various salts
- Dermatology and cosmetics formulations
- Coating premixes
- Taste and odor masking
- Lyophilized Remdesivir Injection for COVID-19
We can help in:
- New Product Development
- Existing Product Improvement
- Enhancing formulations to reduce the cost and wastage
- Training of the staff for wastage control
- Training for formulation development

New Product Development

Existing Product Development

Training of the Staff
Proper Documentation is a major requirement for cGMP compliance. We assist in development of:
- cGMP Documentation
- IQ, OQ, PQ
- SOPs and labeling
- Flow Charts
- Master file presentations
- Dossier Preparation
- Product registration, CTD dossiers, and MSDS preparation

CGMP Documentation
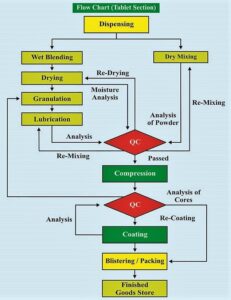
Flow Charts

Dossiers Preparation
We offer comprehensive export services, including:
- Export Documentation
- Pre-registration requirements and stability data
- Site master file and complete company profile preparation
- Audit Preparation

Export Documentation

Site master file

Dossier Preparation

Audit Preparation
We provide assistance in development of automation solutions for:
- Modern ERP development
- Inventory Control
- Payroll Systems
- Attendance, overtime, and biometric systems
- Software for sales, accounts, and procurement
- Record Keeping
- Data retention, backups, and malware protection
- Personal data management and behavior tracking
Modern ERP Development
Attendance overtime biometric
Sales and accounts

Payroll system
Are you ready to collaborate with us on your next project?
Partner with us to bring your vision to life with expert solutions and seamless execution. Let’s work together to achieve success!
